Learning Goals
- Describe how covalent bonds are formed.
- Use dot-cross diagrams to illustrate the bonding in covalent compounds.
- Outline the properties of simple molecules
Covalent Bonding
- Covalent bonds occur between nonmetals, which could be of the same element, or different elements.
- The atoms involved in a covalent bond share one or more electron pair(s).
- Covalent bonding is the electrostatic force of attraction between the nuclei of the non-metallic atoms and their shared pairs of electrons.
- Atoms participate in sharing electron pairs, to gain a stable electronic configuration.
Formation of Covalent Bonds
- We draw dot and cross diagrams with overlapping valence electron shells, to illustrate how covalent bonds are formed. The diagrams below illustrate the formation of a single and a double covalent bond.

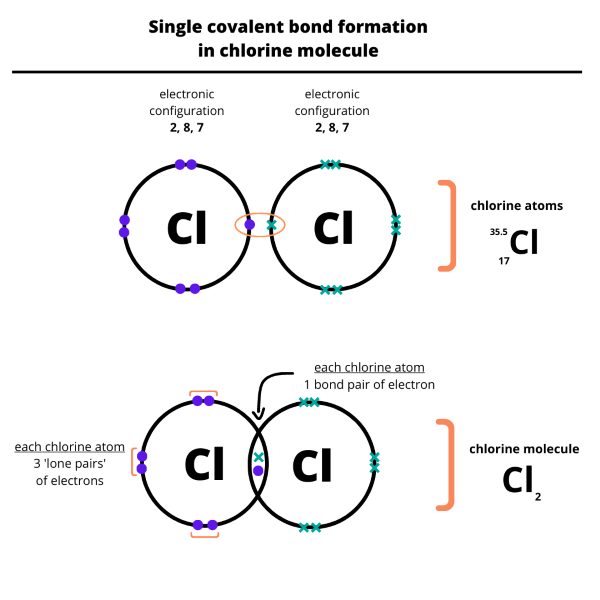
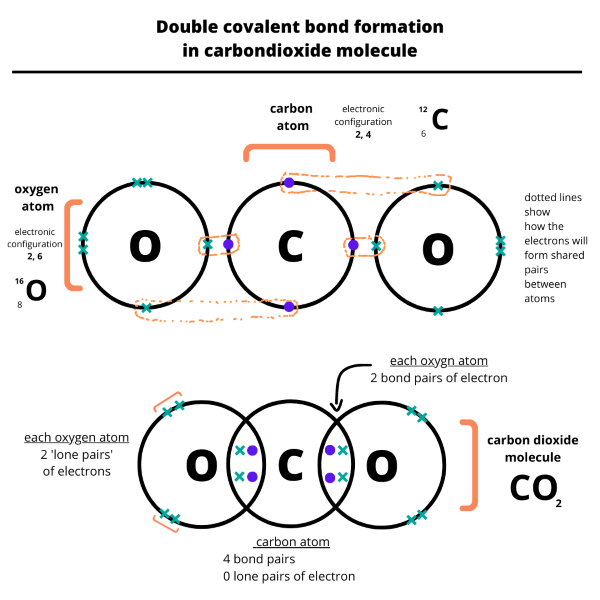
Everything is very open with a clear clarification of the challenges. It was truly informative. Your site is extremely helpful. Thanks for sharing!
Thanks for your blog, nice to read. Do not stop.
I agree!
Good post!